Continuous processing

Continuous adherent cell processing for research and manufacturing
CellRev’s continuous processing platform eliminates many of the limitations and inefficiencies commonly associated with existing cell culture processes.
A proprietary cell detachment process is complemented by a specially designed bioprocess; together, they pose an altogether unique cell manufacturing solution.
Think we can help? Get in touch to explore further.
Scalable adherent cell culture
CellRev combine proprietary cell detachment reagents with a cutting-edge bioprocess to offer customers the premier alternative to both batch and single-cell suspension systems.
CellRev’s bioprocessing platform removes the need to adapt naturally adherent cells so bio-functionality is unaltered and adhesive properties can be retained for DSP. In addition, trypsinisation processes are no longer necessary as CellRev’s proprietary enzymes detach cells within the bioreactor environment.
Research to manufacturing
Continuous cell processing, as highlighted by the FDA, poses a significant opportunity for cellular product developers to improve cost, process stability, and consistency of products.
Continuous processing can;
- Eliminate manual handling and human error
- Increase quality assurance through online monitoring and control
- Reduce manufacturing time and increase efficiency
- Reduce capital costs by using smaller equipment and less manufacturing space
- Respond efficiently in the event of a supply shortage
CellRev support food, therapy and biopharmaceutical manufacturers to overcome USP challenges.
*24 hours doubling time, uptime 90%, seeding density 5000 cells/cm2, final density 80,000 cells/cm2
**40 hours doubling time, uptime 90%, 15% MC Load, seeding density 5000 cells/cm2, final density 80,000 cells/cm2
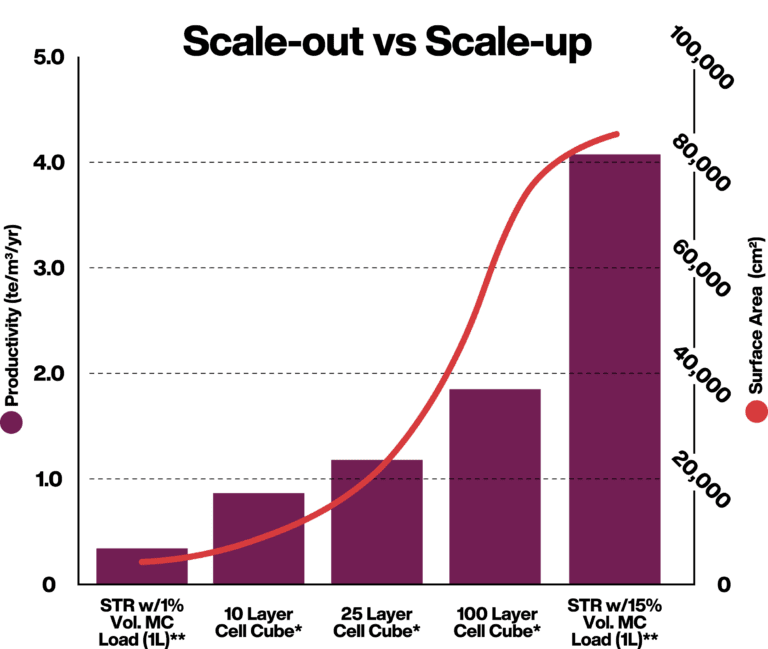
Benefits
3x increase
in bioreactor productivity
40-60% reduction
in labour costs
Up to 95% reduction
on single-use waste consumables
Up to 57% reduction
in CAPEX at scale
Our continuous process
As leaders within their field, CellRev are the first to offer a fully continuous adherent cell culture platform. We develop processes for our customers, commission our continuous production platform, and provide ongoing support to ensure developers and manufacturers capitalise on the full benefit of continuous processing.
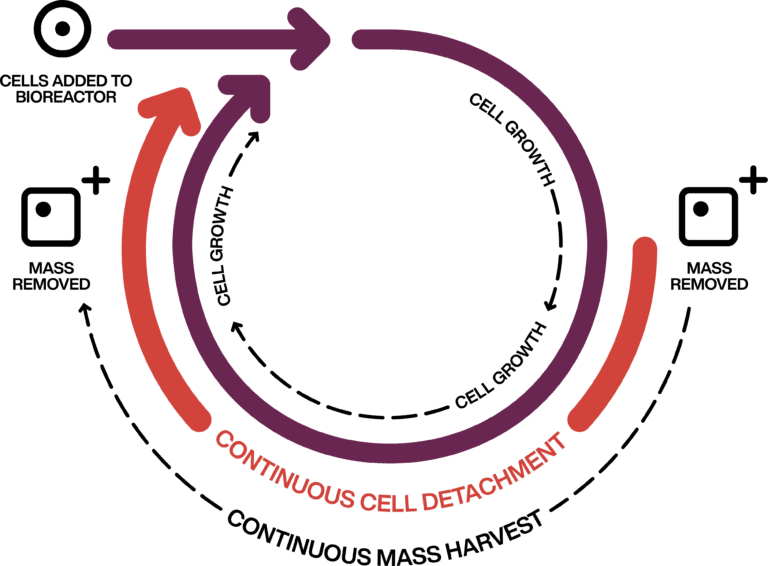
Through a process of balancing adherent cell growth rates with cell detachment parameters, CellRev achieve a steady state within the bioreactor.
Cell densities are held at higher levels over extended periods of time to produce significant yield improvements.
CellRev can demonstrate 60 days of continuous running in their bench-top bioreactors using their proprietary process.
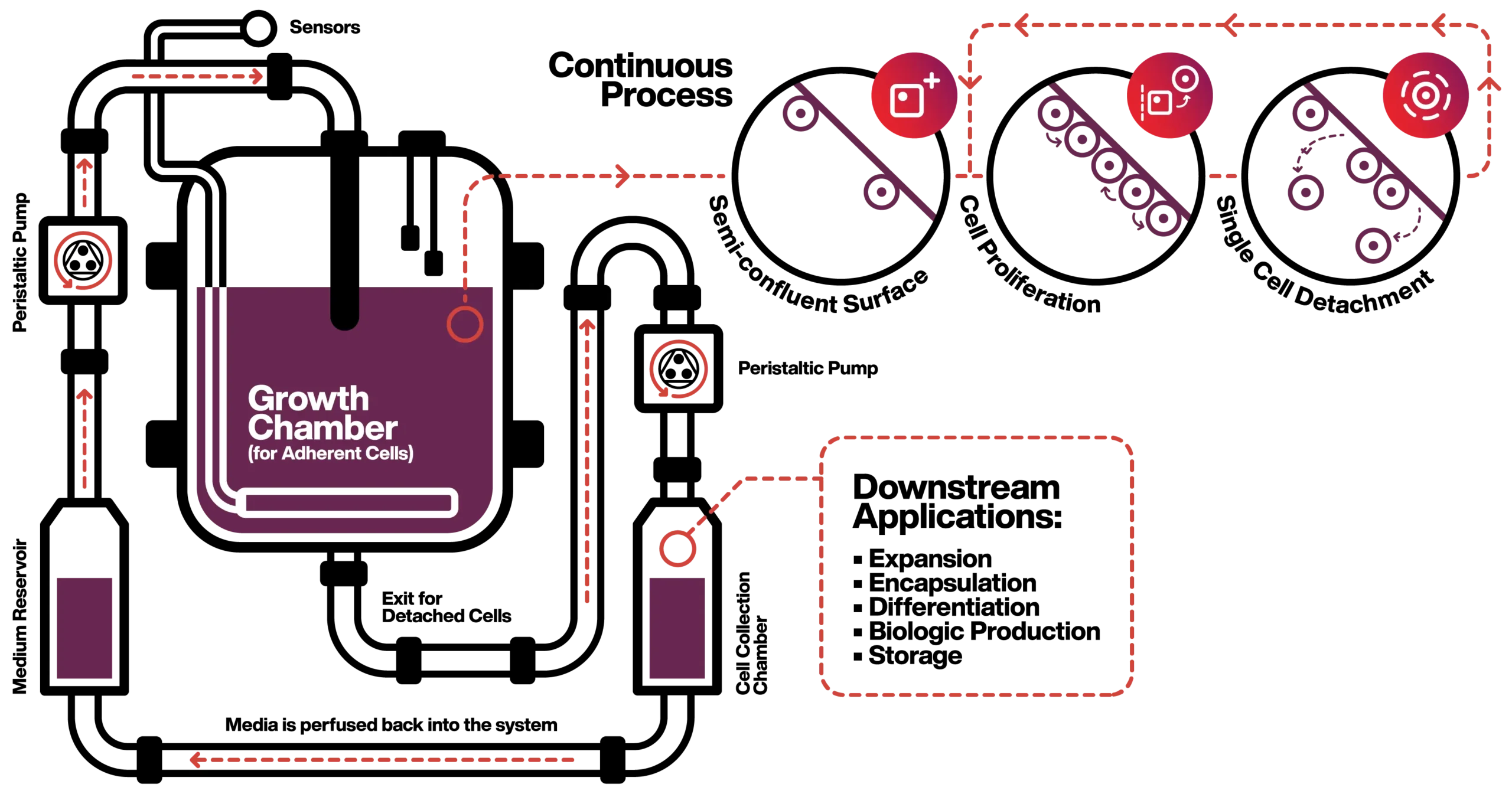
One integrated operation
CellRev has integrated three-unit operations into one automated, closed-loop cell processing platform.
The platform is designed to work with all adherent cell lines. Our Commercial R&D team use their collective bioprocessing know-how to establish the correct parameters for each cell line and associated media formulation. The aim is to match the growth rate of cells with detachment rates to achieve a steady state in the bioreactor. Once set, this protocol can be replicated on every new production run.
This is the critical first step to a highly efficient, continuous process.
Cells are seeded and grown in the bioreactor as per any other cell culture process.
We establish target confluency rates within our process design stage specific to a customer’s requirements.
A proprietary reagent is added to the culture at a predefined concentration to trigger the release of cells to facilitate further expansion. This patented method is unique to CellRev and enables the controlled detachment of adherent cells from a variety of surfaces.
The detached cells temporarily float in suspension prior to collection.
Cells are collected continuously for cell banking or downstream processing. This reduces the need for large scale batch harvesting and facilitates optimal downstream design.
Media is recirculated back into the process. Cells expanded using our platform remain viable, retain their phenotype, and can re-adhere for downstream processing.
Cell Line Studies
We can provide process development support for all adherent cell culture processes. We have already worked with several cell types but continue to explore compatibility and process improvement opportunities with multiple commercial and R&D cell lines. All available case studies can be downloaded below.
Please get in touch if you would like to discuss opportunities to work together on the deployment of our technology or adaptation of your existing platforms.